GBC1
Opening Ceremony / Keynote & Plenary Session
Forum Overview
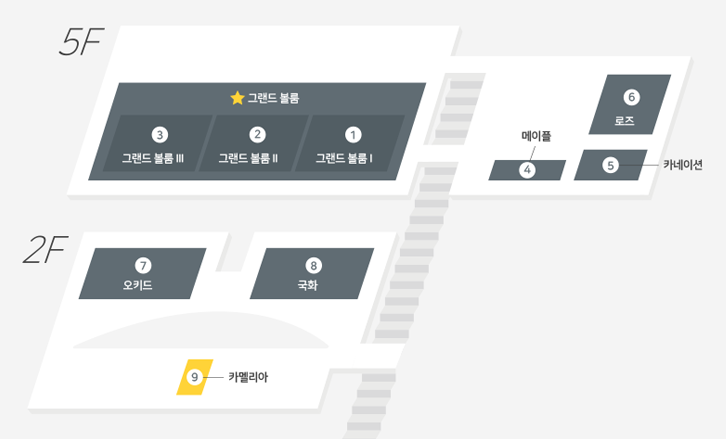
| Date/Time | 9.5 (Mon) 13:30 - 18:00 | Location | Grand Ballroom (5F) |
|---|---|---|---|
| Overview |
New Bio, Beyond Boundaries Our long battle with COVID-19 has driven drastic changes in our core values and ways of life. A “Non-Contact” culture has initiated the advance of digitalization in every industry, facilitating innovation and transitions in the global paradigm and the biopharmaceutical industry is no exception. The paradigm has shifted from treatment-centered to disease prevention and management-centered through data analysis. Artificial Intelligence (AI)-based development of new pharmaceuticals has enhanced the accessibility of treatment for patients and opened more opportunities for the prompt supply of required medicines. Furthermore, we are even arranging proactive countermeasures based on our forecasts of other contagious illnesses and diseases. The Global Bio Conference 2022 aims to understand the present state of the biopharmaceutical industry, which has entered into a new paradigm, and jointly design a bright and sustainable future with regulatory authorities, industrial partners and patients. |
||
Program
GBC2
Vaccine Forum
Forum Overview
| Date/Time | 9.6 (Tue) 09:00 - 13:00 | Location | Grand Ballroom I (5F) |
|---|---|---|---|
| Overview |
Regulatory Strategies for Responding to New Pandemic Diseases through Experience in Developing COVID-19 Vaccine During the two years of the COVID-19 pandemic, regulatory agencies have been making strenuous efforts to address the situation in diverse ways such as through early adoption of vaccines based on new platforms, combinations of new adjuvants and vaccines, and various approaches in response to variant strains, etc. We will look back on these efforts made by regulatory authorities and share strategies to quickly respond to future infectious diseases. |
||
Program
| Time | Program | Speakers | Materials | |
|---|---|---|---|---|
| Chair : Jin Han Kang (The Catholic University of Korea, Medical College) | ||||
| 09:00~09:40 | 40’ | Challenges in rapid response to public health emergencies | Jinho Shin (WHO WPRO) |
|
| 09:40~10:20 | 40’ | Lessons learnt and a look ahead for COVID-19 vaccines | Ralf Wagner (PEI) |
|
| 10:20~10:50 | 30’ | Break | ||
| 10:50~11:30 | 40’ | TBD | Marco Cavaleri (EMA) |
|
| 11:30~12:10 | 40’ | Establishment of Regulatory Strategies through the Development of Covid-19 vaccine | SookMi Hwang (SK bioscience Co., Ltd) |
|
GBC3
Recombinant Protein Products Forum
Forum Overview
| Date/Time | 9.6 (Tue) 14:00 - 18:00 | Location | Grand Ballroom II (5F) |
|---|---|---|---|
| Overview |
The Present and Future of Immune Checkpoint Inhibitors Immune Checkpoint Inhibitors act on the human immune system and serve as a defense system against the onset and progression of cancer. It has become a standard treatment for several malignant tumors. In addition, new pathways and molecules are being explored, and potential treatments are being developed to improve the response and application of immune checkpoint inhibitors. We would like to look into the limitations of the current immune checkpoint inhibitor and its development status along new pathways. |
||
Program
GBC4
Advanced Biopharmaceuticals Forum
Forum Overview
| Date/Time | 9.7 (Wed) 09:00 - 13:00 | Location | Grand Ballroom I (5F) |
|---|---|---|---|
| Overview |
Regulatory Paradigm for Biopharmaceuticals with New Platform Recently, extracellular vesicles therapeutics, mRNA-based personalized treatments, and microbiome therapeutics have garnered attention as promising treatments for the next generation. Advanced Biopharmaceuticals Forum of the Global Bio Conference 2022 will present clinical trial approval cases of extracellular vesicles therapeutics, mRNA-based personalized treatments, and microbiome therapeutics and discuss regulatory issues. |
||
Program
GBC5
Blood Products Forum
Forum Overview
| Date/Time | 9.7 (Wed) 10:00 - 13:00 | Location | Grand Ballroom Ⅱ (5F) |
|---|---|---|---|
| Overview |
Innovative Regulatory Science on Blood Plasma and Plasma Derivatives in the Post COVID-19 Era For the blood, plasma, and plasma derivatives manufacturing industry, the change in production and quality verification technologies are required following the emergence of new infectious diseases and changes in public health and the environment, such as aging, population decrease, climate change, etc. Under the theme 000주제000, the Blood Products Forum will share the recent trends in blood-derived medicinal products and discuss the way forward after the COVID-19 pandemic. |
||
Program
GBC6
Advanced Biopharmaceuticals Policy and Quality Forum
Forum Overview
| Date/Time | 9.7 (Wed) 14:00 - 18:00 | Location | Grand Ballroom I (5F) |
|---|---|---|---|
| Overview |
A Way of Applying Global Harmonization and Data Integrity of GMPs for the Quality Assurance of Advanced Biopharmaceuticals The Advanced Regenerative Bio Act became effective as of August 2020 for the special management system reflecting the features of the advanced biopharmaceuticals, which were previously controlled by the management system for existing biopharmaceuticals. Therefore, we would like to share opinions on reasonable measures to apply GMP and maintain data integrity for the advanced biopharmaceuticals. |
||
Program
GBC7
GMP Forum
Forum Overview
| Date/Time | 9.6 (Tue) 14:00 - 18:00 | Location | Grand Ballroom I (5F) |
|---|---|---|---|
| Overview |
Cross-Contamination Control Strategy for the Expansion of Biopharmaceutical CMO Production During the pandemic, COVID-19 vaccine production through contract manufacturing has been actively carried out, and biopharmaceutical manufacturers around the world have expanded the contract manufacturing organization (CMO) business. Therefore, a strategy for preventing cross-contamination of shared facilities is needed. This year’s GMP Forum will share recent examples and ways of preventing cross-contamination. |
||
Program
GBC8
Regulatory Workshop
Forum Overview
| Date/Time | 9.7 (Wed) 09:00 - 13:00 | Location | Grand Ballroom Ⅲ (5F) |
|---|---|---|---|
| Overview |
Role of Regulatory Authorities and Directions for Advanced Organization All the national regulatory authorities make great efforts to secure the approval, supply and monitoring of safe drug products. This year's Regulatory Workshop will look into the role and the approval & review system of each organization. Furthermore we'll discuss what are the ways and the directions for advanced national regulatory authorities. After the forum ends those industries that pre-applied can have one-on-one meetings with overseas regulators. |
||
Program
| Time | Program | Speakers | Materials | |
|---|---|---|---|---|
| 09:00~09:30 | 30’ | Narantuya Davaakhuu (MMRA, Mongolia) |
||
| 09:30~10:00 | 30’ | Shinichi Okudaira (PMDA, Japan) |
||
| 10:00~10:30 | 30’ | TBD | Cristina Borja (ARCSA, Ecuador) |
|
| 10:30~10:45 | 15’ | Break | ||
| 10:45~11:15 | 30’ | Wilson Bryan (FDA, USA) |
||
| 11:15~11:45 | 30’ | Hui Ming Chua (NPRA, Malaysia) |
||
GBC9
Biopharmaceuticals Future Strategy Forum
Forum Overview
| Date/Time | 9.6 (Tue) 14:00 - 18:00 | Location | Grand Ballroom III (5F) |
|---|---|---|---|
| Overview |
Ways for Fostering and Developing the Biopharmaceuticals Industry Biopharmaceuticals Future Strategy Forum will find agile market entrance strategies for pharmaceuticals with emerging technologies and concepts in the post-COVID-19 era and introduce the government’s efforts dedicated to industry development, such as Global Biomanufacturing Workforce Training Hub plans and goals. By doing so, leaders from government and industry will come together and set out a proper direction for the industry’s future. |
||
Program
GBC10
Regulatory Science Forum
Forum Overview
| Date/Time | 9.6 (Tue) 09:00 - 13:00 | Location | Grand Ballroom Ⅲ (5F) |
|---|---|---|---|
| Overview |
The Role and Direction of Regulatory Science for the Development of Biohealth Industry Regulatory science is getting more attention worldwide as it makes it possible for us to respond to the development in science and technology and even to the new changes in the industrial environment. GBC 2022 Regulatory Science Forum will share strategies and practical cases of regulatory science R&D from global key countries. Furthermore, the role and direction of regulatory science for biohealth industry development in Korea will be addressed. |
||
Program
| Time | Program | Speakers | Materials | |
|---|---|---|---|---|
| 09:00~09:30 | 30’ | The UCSF-Stanford Center of Excellence in Regulatory Science and Innovation: Enabling Research and Education | Kathleen M. Giacomini (UCSF-Stanford CERSI) |
|
| 09:30~10:10 | 40’ | James E. Polli (Maryland CERSI) |
||
| 10:10~10:40 | 30’ | Mark J. Taisey (Amgen) |
||
| 10:40~11:00 | 20’ | Break | ||
| 11:00~11:30 | 30’ | Warren Back (MSD) |
||
| 11:30~12:00 | 30’ | Dong wook Kim (Graduate School of Public Administration of Seoul National University) |
||
| 12:00~12:30 | 30’ | TBD | In-sook Park (MFDS) |
|